- Name: Fluorine Symbol: F Atomic Number: 9 Atomic Mass: 18.998404 amu Melting Point:-219.62 °C (53.530006 K, -363.31598 °F) Boiling Point:-188.14 °C (85.01 K, -306.652 °F) Number of Protons/Electrons: 9 Number of Neutrons: 10 Classification: Halogen Crystal Structure: Cubic Density @ 293 K: 1.696 g/cm 3 Color: Greenish Atomic Structure.
- List the number of protons, electrons, and neutrons in each of the following atoms/ions. For each atom/ion, write a brief (1 – 2 sentences) description of how you determined the number of protons, electrons, and neutrons. A) Calcium cation with a full valence shell and 40 neutrons b) The most common fluorine isotope on Earth (no charge).
- If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine (see Figure below). A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If a fluorine atom gains an electron, it becomes a fluoride ion with an electric charge of -1.
- Fluorine Number Of Electrons Gained Or Lost
- Element Fluorine Protons Neutrons Electrons
- What Is The Mass Number For F
- Fluorine Number Of Electrons In Valence Shell
How many unpaired electrons in a ground state fluorine atom?
Step 4: Find number of bonds by dividing the number of bonding electrons by 2 (because each bond is made of 2 e-) 8e-/2 = 4 bonds. Step 5: The rest are nonbonding pairs. Subtract bonding electrons (step 3) from valence electrons (step 1). 32-8= 24e-= 12 lone pairs. Use information from step 4 and 5 to draw the CF 4 lewis structure.
1 Answer
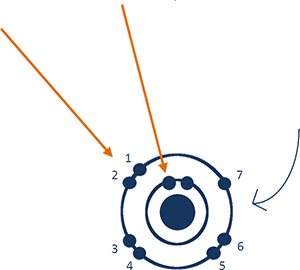

Fluorine has one unpaired electron in the ground state.
Explanation:
Fluorine is in the
The outermost shell of the atom would have
Image from @smarterteacher
The Bohr Model would have
Fluorine Number Of Electrons Gained Or Lost



Element Fluorine Protons Neutrons Electrons
What Is The Mass Number For F
Images from @smarterteacher
Fluorine Number Of Electrons In Valence Shell
Related questions
